News
Europe stands at a turning point in its journey towards establishing a competitive European value chain for batteries. Important steps have been taken in encouraging battery manufacturing plants, only to mention here the inauguration of the first gigafactory by Northvolt in Sweden. Yet, the market demand for batteries continues to surge, fueled not only by the electric vehicle sector but also by other mobility applications and stationary storage needs. The recently launched Quarterly EU Electricity Market Report Q3 ’23 indicates over 600,000 new battery electric vehicles (BEVs) were registered in Q3 ’23, 36% higher than the corresponding quarter in 2022 and counting for 24% market share.
In response to these record demands, the European batteries research and innovation (R&I) community has been dedicated to supporting the establishment of this industrial value chain in Europe, aided by public funding, including by the European Union. Various R&I projects under the umbrella of the BATT4EU Partnership (established under Horizon Europe Programme in 2021), RHINOCEROS included, are sharing forces within the Cluster Hub “Production of materials for batteries from European resources” to address common challenges.
Motivated by the global geopolitical developments, the strategic role batteries play in achieving Green Deal objectives and the ever-evolving nature of battery technologies, Europe recognises the critical need for strategic alignment among stakeholders. Replacing the BATT4EU SRIA of 2021 and the Batteries Europe SRA of 2020, the 2024 SRIA outlines key strategic actions that the European Batteries R&I Community will undertake to advance collaborative research projects facilitated by the BATT4EU Partnership. Different from the previous strategic agendas, the 2024 roadmap goes beyond specific chemistries, leveraging also the power of disruptive (digital) technologies to advance research across all battery types, including material science, manufacturing and recycling processes.
The new agenda draws on the roadmaps published by Batteries Europe and Battery 2030+, compiling inputs from numerous European battery experts, offering recommendations on short, medium, and long-term objectives. It emphasises the need for coordinated action not only at the European level but also within national and regional programmes.
The 2024 SRIA points to the following six imperatives which are necessary to set the foundations and support a competitive battery value chain in Europe:
• Ensure that (BATT4EU) research results reach gigafactories and the markets, through pilots, demonstrators and improved decision making aided by digital tools.
• Increase the strategic autonomy of Europe by reducing the reliance on foreign critical raw materials by supporting local and circular supply chains and support research into different battery chemistries, including sodium-ion technologies.
• Improve battery affordability to accelerate the green transition and keep the European industry competitive by improving batteries based on materials that are more abundant and pushing for better integration into end-use applications.
• Improve the flexibility of battery manufacturing and recycling systems to reduce lock-in effects and respond quickly to changes in a rapidly developing industry.
• Implement a safe and sustainable by design framework for batteries, which plays to European strengths, and which will help reduce emissions and use of substances of concern, improve safety and allow for the integration of smart functionalities.
• Support the continuity of excellent European battery research and academic-industrial cooperation by improving access to research facilities and pilot lines, use research projects to build up a skilled workface, and by avoiding gaps in research through continued funding, which will bind talented researchers to Europe.
Interested in finding out more information about the recently released SRIA?
BATT4EU Partnership is organising a webinar on 20 March 2024, between 10:00 and 11:30 Brussels time. The aim is to present the official document and to host engaging discussions with the experts behind this publication who will explain how this document will redefine the dynamics for the European battery sector.
Register here
RHINOCEROS attending Shifting Economy Week
From 21 to 25 November 2023, the city of Brussels hosted the Shifting Economy Week, an annual event dedicated to showcasing transformative projects that aim to pave the way to an economy that is low-carbon, regenerative, and equally circular. The 2023 exhibitors’ line-up included, among other regional stakeholders, our partner Watt4Ever (W4E), industrial partner specialised in the development of innovative solutions for energy storage and management. W4E leveraged its presence at Shifting Economy Week to to raise awareness about the importance of circular economy principles in the context of the battery industry.
During the same event, W4E’s CEO, Aimilios Orfanos, was invited to speak at the BeCircular conference, an event dedicated to presenting concrete examples of circular economy approaches put in place by Brussels-based companies. He shared insights from W4E’s experience in developing second-life battery systems for electric vehicles, emphasising their potential benefits in terms of environmental impact and cost savings. Simultaneously, the CEO also highlighted the challenges faced by the industry in implementing circular business models, including regulatory barriers and market incentives.

RHINOCEROS at its second participation at Circular Wallonia Days
A few days after attending Shifting Economy Week, W4E represented the RHINOCEROS project at the Circular Wallonia Days, held on 13 and 14 December 2023. Centred around advancing the circularity of the batteries value chain, the event brought together stakeholders from academia, industry, and government to discuss strategies for improving the sustainability of battery production, use, and disposal. The focus topics covered recycling technologies, supply chain transparency, and policy measures to support the transition to a circular battery economy.
© Photo credits: Watt4Ever
Despite a different objective, the RHINOCEROS project partners have shown growing interest in the Digital Battery Passport, an initiative of FREE4LIB, a sister project from the Cluster Hub “Production of raw materials for batteries from European resources”. This collaboration shows our commitment to contributing to the European battery community through the exchange of knowledge and experience.
The FREE4LIB workshop had a three-fold objective, including a brief presentation of the preliminary results of the battery passport concept development, the outline of the implementation challenges and potential follow-ups of industrial scale-up, and the clear differentiation between battery second use (B2U) versus recycling. The event drew approximately 50 participants from various segments of the battery value chain, which ensured a comprehensive and multifaceted perspective of the subject matter.
The introductive session presented the FREE4LIB project, briefly highlighting past achievements and focusing mainly on the remaining activities outlined in the workplan. The following session was led by Julius Ott (industrial engineer with expertise in circular economy at Karl-Franzens-Universität Graz). During the past year, researchers at Univ. of Graz worked on finalising data collection and processing related to the development of a data model of the digital passport platform which aims to close the information gap between beginning-of-life (BoL) and end-of-life (EoL) battery lifetime. This interactive session turned out to be an appropriate opportunity for researchers at Univ. of Graz to present the outcomes of their data collection and handling, and to evaluate their relevance within the reality portrayed by the workshop attendees.
Participants, predominantly familiarised with the EU-funded battery projects, confirmed the findings reported by Univ. of Graz. However, they also raised concerns about data sharing. The outcomes of the interactive session, complementing prior research, will serve as valuable guidance for the FREE4LIB project in implementing the battery passport within their project.
For additional background information on the digital passport developed by FREE4LIB, please refer to previous articles.
On Thursday, 16 November, during the 2023 edition of the Raw Materials Week, the twelve EU funded projects that constitute the Cluster Hub ‘Materials for batteries’ gathered for their annual event in Brussels.
The Cluster Hub has been initiated last year during the 7th edition of the Raw Materials Week. The main objective of the meeting was to meet and discuss the latest developments in the participating projects as well as the new challenges and opportunities discovered through the projects’ lifetime. Nader Akil, Operations Manager at PNO Innovation, inaugurated this second edition outlining the motivation behind the hub’s establishment. He underlined the positive reception and sustained interest from various stakeholders keen on joining this initiative.
Discover and/or rediscover the first edition of the Cluster Hub workshop
Co-organised by RELiEF, EXCEED, ENICON and RAWMINA, the event was also the opportunity to welcome the four new members of the Cluster (EXCEED, RAWMINA, METALLICO and CRM-geothermal). the workshop gathered nearly 100 organisations driving the production and the recycling of raw materials for battery applications from primary and secondary resources.
Building on the initial objective of creating an environment that could foster knowledge exchange on different approaches for the recycling and recovery for battery applications, the event focused on three major topics that depict the transversality characterising the projects: the raw materials through research and science, the roles and challenges of industry and market for raw materials, and the raw materials under the scope of sustainability, durability and social acceptance. During this annual meeting, an interactive session led by Anish Patil from TechConcepts and representing the RELiEF project had the objective of Mapping the European battery material recycling landscape – more details to be found below, in the section referring to the interactive session.
Research and science unlocking new opportunities in raw materials
The first session was moderated by Sonia Matencio from LEITAT, representing the RAWMINA project. This session had the objective of discussing the raw materials through research and science, under the scopes of mining, refining, processing as well as the battery data. Sonia introduced this topic under the scope of RAWMINA, explaining the integrated innovative pilot system for Critical Raw Materials recovery from mine waste in a circular economy context. To this end, Christophe Aucher, from LEITAT as well, highlighted the need on an open battery passport system to better reflect and account for any adaptations that might be required due to the changing regulatory landscape.
Sonia welcomed afterward Brecht Dewulf from KU LEUVEN and representing ENICON, who discussed the sustainable processing of Europe’s low grade sulphidic and lateritic Ni/Co ores and tailings into battery grade metals. The idea behind this was to show all the potential of Ni/Co resources for Europe.
Xochitl Dominguez from VITO concentrated her speech on gas-diffusion electrocrytallisation (GDEx), a crucial topic for the projects LiCORNE and RHINOCEROS she works with. GDEx is an electrochemical process of reactive precipitation of metals in solution with oxidising or reducing agents produced in-situ by the electrochemical reduction of a gas, in a gas-diffusion electrode. This was followed by Katrin Kieling from GFZ Potsdam, working there for the CRM-geothermal project and shortly explained the challenges of extracting critical raw materials from geothermal fluids. To conclude this first session, Sandra Pavón from Fraunhofer IKTS explained the demonstration of battery metals recovery from primary and secondary resources through a sustainable processing methodology in the METALLICO project.
Discover presentations from Session 1
Insights from stakeholder perspectives: Interactive session on key EU Policies and priorities
The annual meeting followed its course with an interactive session led by Anish Patil, which scrutinised stakeholders’ perspectives on the Green Deal Industrial Plan, Net Zero Industrial Act, Critical Raw Materials Act and the European Battery Regulation 2023. Mentimeter facilitated this interactive session, engaging the audience to explore how these policies intersect, complement each other, and identify critical measures and incentives for achieving their objectives.
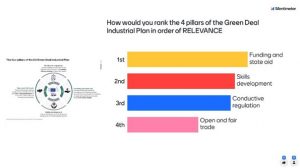
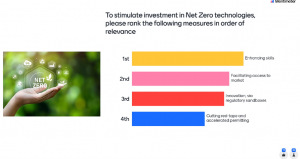
Over 30 persons participated in the live-poll proposed, which results display the priority to be set on funding and state aid regarding ranking the four pillars of the Green Deal Industrial Plan in order of relevance (followed by skills development, conductive regulation, and open and fair trade). Another major topic regarding the stimulation of investment in net Zero technologies, the majority of answers placed the ‘enhanced skills’ as first priority, shortly followed by facilitating the access to the market.
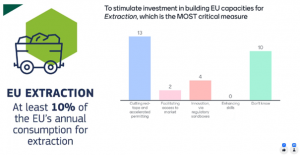
Lastly, the participants were divided regarding the critical measures to implement in the EU to stimulate investment in building domestic capacities for extraction of critical raw materials (CRMs). Although the majority opted for ‘cutting red-tape and accelerated permitting’, approximately half of the answers evoked uncertainty, which emphasised one more time the need to engage with policy makers as external stakeholders in all projects.
Navigating the nexus: industry challenges, market dynamics, social acceptance and sustainability
This interactive workshop was followed by two sessions, which aimed at discussing the challenges and opportunities of raw materials within the frame of industry and market, as well as the social acceptance, sustainability, and durability.
Alan Gonzalez from PNO Innovation Begium, representing LiCORNE, moderated the industry part, whereas Sam Hoefman from RELiEF moderated the last session on social acceptance, sustainability, and durability. Distinguished panellists took the stage to engage in debates on various topics.
Edvarts Emerson, Production and Testing Engineer at Watt4Ever, presented his work on the benchmark depository of 2nd life use of lithium in batteries, acceptance criteria and guidelines, work developed within the RHINOCEROS project. Benjamin Wilson, representing the RESPECT Project, displayed Aalto University’s work advancing efficient, sustainable, innovative and safe battery recycling processes in the EU. Laura Kainiemi from LUT University, representing the RELiEF Project, Konstantinos Komnitsas from the Technical University of Crete (TUC), on behalf of EXCEED, and Vitor Correia from INTRAW for the CRM-geothermal project, collectively debated the role and impact of social acceptance among affected communities, the importance of triggering new dialogues on responsible mining activities, and the joint involvement of regional, national and European authorities, academia, industry partners, and citizens in shaping these initiatives.
A big thank you to all participants for this co-creative and very constructive and inspiring meeting.
Discover presentations from Session 2
Discover presentations from Session 3
The modified Black Mass (BM) obtained in Task 4.4 – Reactive milling for the production of metallic black mass (MBM), as well as the BM supplied by partner ACCUREC (ACC) were leached under different conditions to extract lithium (Li) salt.
The BM provided by ACC consists of graphite, as well as transition metals such as nickel (Ni), manganese (Mn), cobalt (Co), along with their respective oxides and impurities – copper (Cu), iron (Fe), and few fluorinated compounds. Li is present in the form of lithium carbonate [Li2CO3] and lithium aluminium oxide [LiAlO2 ]. However, breaking off LiAlO2 to a soluble Li-salt and removing fluoride contamination is a challenging process. To overcome this, the BM was heated with calcium hydroxide in deionised water for one hour and then filtered.
Illustrated in the next figure, LiAlO2 decomposes during heating and transforms into insoluble calcium aluminium hydroxide. Dissolved fluoride anions are caught by Ca2+ ions and precipitate as calcium fluoride. The soluble part only contains Li- and Ca-salts. The latter can be transformed into calcium carbonate and removed with a second filtration step.
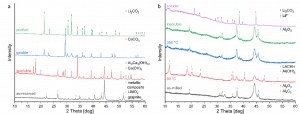
X-ray diffraction patterns of leaching and purification process. a) black mass supplied by ACC and treated with calcium hydroxide. b) aluminium system of modified black mass
The second BM from partner TES, obtained after ball milling, consists of graphite, the metallic composite, lithium oxide and the oxidized reducing agent, along with some impurities like Fe and fluorinated compounds.
Lithium extraction after ball milling
In the aluminium system, the extraction of lithium salt poses certain challenges. Due to the tendency of residual aluminium powder to ignite when in contact with air, this requires cautious handling. During milling lithium aluminium oxide is formed, and in the basic solution, a significant amount of aluminium hydroxide dissolves and reacts with lithium hydroxide and CO2, resulting in the formation of a poorly soluble compound called LACHH. To address this problem, the suspension is heated to 90°C to reinforce the reaction between aluminium hydroxide and lithium hydroxide. The LACHH formed can be decomposed at 350°C into insoluble aluminium oxide and soluble lithium carbonate, which can be separated through filtration.
Lithium extraction in the calcium system, on the other hand, is a relatively straightforward process. The milled powder shows low reactivity towards both oxygen and water. Scientists successfully extracted 98% of lithium from the black mass; however, calcium hydroxide was also dissolved in the process. Subsequent purification steps led to the isolation of lithium carbonate with a purity of 93% in a yield of 75%. The main impurities are shown in the figure displayed below.
In the upcoming months, researchers will work to improve the leaching conditions towards a lower reagent and water consumption, while obtaining a higher purity of resulting lithium salt and lower lithium loses during purification steps.

Impurity cations in isolated lithium carbonate from the calcium system of modified black mass
During the second semester, researchers from KIT further studied and improved the conditions for the mechanochemical transformation of Black Mass (BM). With the BM supplied by ACC is already in a reduced state, the focus now shifts towards reducing the BM provided by partner TES.
This BM consists mostly of nickel-manganese-cobalt (NMC) cathode material and graphite, and it was found to cause a longer reaction time. It is expected that the graphite exfoliates during milling and creates thin protective layers around NMC particles and the reducing agent, slowing down the reaction kinetics.
Using variations of milling parameters like ball-to-sample ratio or the type and amount of reducing agent, researchers optimised the process towards the fastest kinetics. During the investigation, no intermediate reduction to transition metal oxides in lower oxidation states was observed. However, the full reduction of the respective part to the metallic composite occurred.
The operation took place in a shaker mill, and it requested an excess of 3.3 equivalents of Aluminium (Al) towards NMC was required to attain a complete reduction within a reasonable time. This transformation was accompanied by the formation of aluminium carbide and the presence of residual fine aluminium powder which caused a self-ignition hazard when the milled powder came into contact with air.
In contrast, when using calcium as a reactive agent, researchers observed a fast reaction but they also reported a strong dependency of reaction kinetics on calcium size. The hazard of self-ignition when exposed to air was limited in this case by using stoichiometric amounts.
In addition, the research team has been conducting preliminary experiments for the scale-up process using a planetary mill. It was observed that the choice of the milling type has a significant impact on the reaction kinetics. Attempts to crush calcium pieces proved to be unsuccessful, therefore not initiating any reaction. However, when using aluminium, reactions occurred much faster.
In the months ahead, the research team working in WP4 will continue to investigate the milling parameters for the planetary mill.
Find out more information about the activities developed in WP4 in the article Reactive milling for the production of metallic black mass (MBM) – Rhinoceros project (rhinoceros-project.eu)
Read more about the black mass leaching operations in the article Materials extraction and direct routes for the synthesis of electrode materials: Recovery of Lithium as battery grade materials
Three dimensional (3D) Scanning of Battery Packs
Following the manual dismantling of various battery packs during the first six months of project, researchers at University of Agder (UiA) have developed a semi-automated process to address the diverse nature of battery packs. Their advanced robotic system can estimate the size of individual components of a battery pack. Afterwards, using different angles, it identifies optimal locations to capture precisely 3D images of these components, thus ensuring no detail is missed through this comprehensive scanning.
Using different perspectives, this thorough scanning process is further translated into a list of point clouds. Applying sophisticated algorithms, these point clouds are later combined and merged into a solid mesh component. This process is repeated individually for each new component which needs to be scanned.
Beyond geometric characteristics
While geometric characteristics are important, they provide even greater value when combined with other physical attributes and interconnection data of the components. The researchers have successfully documented these details, resulting in a rich digital repository of the battery pack. With the establishment of this detailed digital repository, the focus is now shifting towards its applications, where the primary goal is to automate the disassembly sequences.
Simultaneously, the team is also focusing on the automatic characterisation of battery packs and modules. Significant efforts are channeled towards creating a robust digital simulator, which will serve as a platform for training and rigorous testing of the disassembly planner – currently under development.
Innovation in disassembly tools
At the same time, the research team involved in work package 3 have been working on improving the disassembly operations tools. The results reported positive feedback, with several tools already successfully tested in lab environment. This is a significant step towards the fully automated disassembly process.
A noteworthy development is the automation of the non-destructive disconnection of cables. This procedure is essential as it stands as the second most frequent operation in battery pack disassembly, just after the unscrewing operation.
You can read more about previous activities developed in work package 3 in the article ‘Manual dismantling of a battery pack‘.
On Monday, 10 July 2023, the Council of the European Union adopted a new regulation that strengthens sustainability rules for batteries and waste batteries. This regulation covers the entire life cycle of batteries, ensuring their safety, sustainability and competitiveness from production to reuse and recycling.
Read the official press release
Recognising the vital role batteries play in the decarbonisation process and the transition towards zero-emission mobility, Teresa Ribera, Spanish Minister for the Ecological Transition reinforced the Presidency’s commitment to supporting comprehensive regulation encompassing all types of batteries. This includes waste portable batteries, electric vehicle batteries, industrial batteries, starting, lightning and ignition (SLI) batteries primarily used in vehicles and machinery, as well as batteries for light means of transport like electric bikes, e-mopeds, and e-scooters.
“At the same time end-of-life batteries contain many valuable resources and we must be able to reuse those critical raw materials instead of relying on third countries for supplies. The new rules will promote the competitiveness of European industry and ensure new batteries are sustainable and contribute to the green transition.”
| Teresa Ribera, Spanish Minister for the Ecological Transition
To foster a circular economy, the regulation establishes requirements for the end-of-life phase, including collection targets and obligations, material recovery targets, and extended producer responsibility. Dedicated collection objectives for waste batteries used in light means of transport will be implemented, aiming at 51% by the end of 2028, respectively 61% by the end of 2031. Furthermore, the regulation sets mandatory minimum levels of recycled content for industrial batteries, SLI batteries and electric vehicle batteries. The following initial values have been established:
- 16% for cobalt
- 85% for lead
- 6% for lithium
- 6% for nickel
Additionally, batteries will also be required to hold documentation proving their recycled content.
To improve the functioning of the internal market for batteries and ensure fair competition, the regulation introduces safety, sustainability, and labelling requirements. It includes provisions for battery labelling and information disclosure, including details on battery components and recycled content. Additionally, an electronic “battery passport” and a QR code will be implemented to enhance traceability and transparency. These labelling requirements will take effect by 2026, while the QR code implementation is expected by 2027, providing member states and manufacturers with ample time to prepare.
This new regulation aims to mitigate environmental and social impacts throughout the battery’s life cycle. By establishing strict due diligence rules for operators, the EU is ensuring operators are bound to verify the source of raw materials used for batteries placed on the market. However, the regulation provides for an exemption for SMEs from the due diligence rules.
After its signature by the Council and the European parliament, the new regulation will be published in the EU’s Official Journal, expecting to enter into force 20 days after.
RHINOCEROS project in the current legislative framework
Launched in 2022, the RHINOCEROS project fits within the current framework recently adopted by the Council of Europe under the Spanish Presidency. Designed to support the raw materials supply, the RHINOCEROS project will demonstrate a smart sorting and dismantling robot at TRL6, enabling the automation of a battery repurposing production line. When direct reuse and repurposing of batteries is not possible, RHINOCEROS will investigate several ground-breaking circular recycling routes aiming at the recovery of all materials present in LIBs (e.g., metals, graphite, fluorinated compounds, electrolytes, polymers, and active materials).
A first set of conclusions stemming from the research of our partners generated a database and the parameters for module selection, which will further facilitate the development of electric vehicles 2nd life batteries.
Read more in the article “Acceptance criteria and guidelines for 2nd life prone LIBs”
The infographic can be accessed on the Council of the European Union’s website using this link.
Aiming towards a zero-waste strategy for the recovery of metals from battery refining waste waters, LEITAT is working on the development and evaluation of novel polymer inclusion membranes (PIM). PIMs are a type of liquid membrane in which the liquid phase, the extractant, is held within a polymeric network. The interest in these membranes has been growing exponentially over the past few years as an alternative separation technique to conventional solvent extraction.
Work during the first six months has focused on the evaluation of different extractants for the target metals: lithium, manganese, cobalt and nickel. Researchers established a liquid-liquid extraction protocol based on two different processes in which the target metal is extracted and recovered separately. During the extraction step, a specific carrier compound selective towards the target metal separates an amount of it from a feed metal solution. The recovery of the metal takes place in the second process, where a stripping solution is employed to recover the metal previously extracted through the carrier. Initial PIMs containing the most efficient extractants have been prepared, characterised and are currently evaluated. The featured image depicts the continuous procedure used to test the synthesised PIMs.
The current industrial pre-treatment and downstream processes (e.g., pyrolysis, calcination, etc.) are still inefficient and have significant limitations. Plastics and electrolytes sacrificed in the initial stages of the recycling process are overlooked when it comes to their recovery. Within the RHINOCEROS project, Chalmers University [CHA] is working on recycling of ignored content of the LIBs waste, electrolyte and polymeric materials by developing an innovative process based on Supercritical Carbon Dioxide (sc-CO2) technology.
Due to its environmental friendliness, non-toxic, low cost and straightforward processing features, the sc-CO2 technology has been attracting both scientific and industrial interest. With consistent leverage over other processes, sc-CO2 technology is already widely used in various industries including food, cosmetic and pharmaceutical industries [i.e., to decaffeinate coffee or tea, extract vegetable oils etc.] Although it has many applications, its use in battery recycling was recently discovered and the Chalmers research group is one of the pioneers in this field.
Within the project’s framework, CHA researchers are targeting the development of the sc-CO2 extraction process which will selectively recycle the electrolyte and the polymeric material from the LiB waste. The electrolyte, binder, and separator will be recycled in subsequent steps and purified to reuse in the battery industry. For this purpose, several critical process parameters such as pressure and temperature are investigated to achieve high recycling efficiencies under feasible conditions.
Electrolyte recovery
The electrolyte in the LiB is a complex system usually composed of a conductive salt dissolved in a matrix of various solvents and additives. The most recent results reported by CHA on sub- and sc-CO2 research show that at low pressure and temperature conditions, the non-polar electrolyte components (almost 66% of the electrolyte) were selectively recovered without the generation of toxic gas emissions, which are typically generated by thermal recovery processes originated by the decomposition of the thermally unstable conductive salt.
In this recycling step, the polar electrolyte components are left in the sample as residues and a subsequent recycling step using suitable cosolvent is required for their selective extraction. During the upcoming phases, researchers will aim to recover the electrolyte completely, including conductive salt and solvents.
In this recycling step, the polar electrolyte components are left in the sample as residues and a subsequent recycling step using suitable cosolvent is required for their selective extraction. During the upcoming phases, researchers will aim to recover the electrolyte completely, including conductive salt and solvents.
The selection of the co-solvent is critically important not only for effective recycling but also for the sustainability and the feasibility of the developed processes. To assess the suitability of the sc-CO2 process, its environmental impact and economic competitiveness, CHA researchers explored also other solvents, which allowed them to select the most promising candidates for future research.
In the upcoming months, CHA will study the effects of co-solvent modified sc-CO2 system parameters on the recycling efficiency of PVDF and the structural properties of the recycled material. Researchers will carry out intensive characterisation studies to clarify the changes in the material structure, to determine the quality, and to optimise the process to reach the reusability goal.